We Keep Growing
We are the official Turkey office of OMC MEDICAL, one of the well-established companies in the UK.

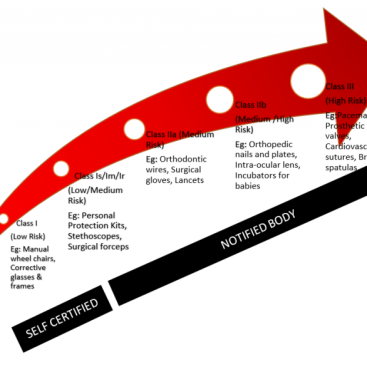
ARE YOU READY FOR THE MDR - IVDR PROCESS?
As of May 26, 2021, a brand new era has begun!
CE PRODUCT CERTIFICATION
Let's prepare your technical files, make your system applicable and have a smooth audit experience together.
ISO 13485:2016
Medical Quality Management System
International Medical Product Certification and Audit Experience

MDR / IVDR: IMPORTER & DISTRIBUTOR
If you are an Importer and Distributor, What you will encounter in the MDR & IVDR Process
Get Information for what you need to do
ISO 13485 Consulting for Medical Devices
Companies producing Medical Devices should appoint an experienced ISO 13485 Consultant...
Risk Management
Let’s review the background of ISO 14971. As a medical device manufacturer, the first thing you need to do...
Preparation of CE Technical File
The MDR 2017 / 745 Annex II Medical Device Technical File is a clear, well-organized, easily searchable...
Internal Audits, Supplier Audits
We provide audit expertise that can more effectively drive change and improvements. Your internal audit...
Medical Device Single Audit Program
MDSAP (Medical Device Single Control Program) is one of the (very) few harmonization programs...
EU MDR - IVDR
Determining the Medical Device Classification and related classification rule is the first step in the EU CE...
CE Product Certification
With the introduction of IVDR, it turned out that bringing IVD devices to the EU market has turned...
FDA Quality System Regulation
With our US FDA 510(k) Consulting service, we help you go through the entire process by thoroughly...
Validation
Process Validation is performed to ensure consistent delivery of quality products that meet predeter...
Biocompatibility – Biological Evaluation
A medical device is not expected to evoke any biological response in the patient when used as...
Clinical Evaluation
Clinical evaluation is required for medical devices under medical device directive 93/42/EEC...
Clinical Data Evaluation
What are the sources of documents and data used in Clinical Evaluation?
PMS & PMCF
Regardless of the classification of the medical device, Post-Market Inspection is necessary and...
SSCP
The Safety and Clinical Performance Summary is to provide healthcare professionals with...
GAP Analysis In Healthcare
Gone are the days when the family doctor made house calls. Welcome to the modern healthcare...
UTS and EUDAMED Records
Product Tracking System (ÜTS for short), MDR, EUDAMED are among the topics that the medical...
Your Regulatory Expert
With 20 years of experience in manufacturing, inspection and R&D in the medical device industry, we will help you develop your product in accordance with worldwide regulatory requirements.
MedENvolve Consultancy







































COMMENTS
Customer Satisfaction
“
DO YOU HAVE A QUESTION?
Frequently Asked Questions
1) When should I start preparing for the EU MDR?
Medenvolve Consultancy2023-12-07T19:02:06+03:00Two years ago when the announcement of the new regulation was first made. Call us today at 05447772038 if you need help.2) Will these changes affect me?
Medenvolve Consultancy2023-12-07T19:02:37+03:00If you market your device in the European Union (EU) under the MDD, note that there is no historical allocation of devices under the MDR. In addition, your device classification may have changed and may now require clinical assessment and renewal of the technical dossier.3) Is my product obliged to comply with the EU MDR?
Medenvolve Consultancy2023-12-07T19:03:39+03:00The new EU Medical Device Regulation has broadened the scope of EU MDR applicability. Some products that were not previously recognized as medical devices are now covered by the medical device regulation. The newly added products include:- Contact lenses or other substances intended to be inserted into or on the eye.
- Products intended to be inserted, in whole or in part, into the human body by surgically invasive means for the purpose of altering the anatomy or fixation of body parts, except for tattoo products and piercings.
- Substances, combinations of substances or items intended for use for filling the face or other dermal or mucous membranes by subcutaneous, submucosal or intradermal injection or other introduction, except for tattooing.
- Equipment intended for use to reduce, remove or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty.
- Equipment that emits high-intensity electromagnetic radiation (e.g. infrared, visible light and ultraviolet) intended for use on the human body, including monochromatic and broad spectrum, such as coherent and incoherent sources, lasers and intense pulsed light equipment for skin resurfacing, tattooing or hair removal or other skin treatment.
- The skull to alter neuronal activity in the brain.
4) What is the first step I should take now?
Medenvolve Consultancy2023-12-07T19:04:10+03:00We know that the EU is a huge market for any medical device manufacturer and losing this revenue will have a negative impact on your business. We recommend you start by conducting a gap analysis of your current quality system and documentation. Contact us now using the form below. Our team of EU MDR experts will help you plan your next steps.
Contact Us
Consultancy Request
ARE YOU LOOKING FOR
Experienced Consultant?
Contact us right now
Are You Ready for MDR - IVDR Process?
As of May 26, 2021, the European Union is implementing stricter rules on Medical Devices (MDR). From May 26, 2022, the new regulations will also apply to In Vitro Diagnostic (IVDR) Regulations. Find out what the consequences are for you as a distributor or importer.
- EUDAMED Regist.
- UDI-DI, UDI-PI
- Vigiliance System
- Compliants
- Verifications
Get Legal Help
Lorem ipsum dolor sit a met, consectetur adipiscing elit.
Get Legal Help
Lorem ipsum dolor sit a met, consectetur adipiscing elit.
Get Legal Help
Lorem ipsum dolor sit a met, consectetur adipiscing elit.
MEDENVOLVE CONSULTANCY
Our Services
AB MDR-IVDR
Medenvolve Consultancy2023-11-21T21:26:31+03:00Biyouyumluluk – Biyolojik Değerlendirme
Medenvolve Consultancy2023-12-07T18:22:04+03:00CE Belgelendirme, Tıbbi Cihaz Danışmanlığı, MDR ve IVDR: Ürünlerinizi Başarıyla Piyasaya Sunun!
Medenvolve Consultancy2023-12-05T23:09:00+03:00FDA Kalite Sistem Regülasyonu
Medenvolve Consultancy2023-12-05T16:47:22+03:00İç Tetkikçiler, Tedarikçi Denetimleri
Medenvolve Consultancy2023-12-08T18:40:41+03:00Klinik Değerlendirme
Medenvolve Consultancy2023-12-08T18:41:51+03:00Klinik Veri Değerlendirme
Medenvolve Consultancy2023-11-21T20:15:44+03:00MDR/ IVDR : İthalatçı & Distribütör
Medenvolve Consultancy2023-11-21T22:00:24+03:00Medikal Cihaz Dizayn, Proses ve Yazılım Validasyon Danışmanlığı
Medenvolve Consultancy2023-11-21T20:44:05+03:00PMCF
Medenvolve Consultancy2023-11-21T21:48:00+03:00PMS
Medenvolve Consultancy2023-11-21T20:10:55+03:00Sağlık hizmetlerinde GAP analizi
Medenvolve Consultancy2023-12-08T18:43:20+03:00SSCP
Medenvolve Consultancy2023-12-08T18:44:38+03:00Tıbbi Cihaz Tek Denetim Programı (MDSAP)
Medenvolve Consultancy2023-11-21T21:29:34+03:00Tıbbi Cihazlar için CE Teknik Dosya (MDR)
Medenvolve Consultancy2023-12-05T23:10:45+03:00Tıbbi Cihazlar İçin ISO 13485 Danışmanlığı
Medenvolve Consultancy2023-12-08T18:48:42+03:00Tıbbi Cihazlar İçin ISO 14971 Risk Yönetimi
Medenvolve Consultancy2023-12-08T18:47:13+03:00ÜTS ve Eudamed Kayıtları
Medenvolve Consultancy2023-12-05T23:12:20+03:00USEFUL INFORMATION
News from the Sector and Us
IVDR Transition Process
Medenvolve Consultancy2023-12-08T20:07:43+03:00Regulation (EU) No 2022/112 of the EUROPEAN COMMISSION of 25 January 2022 amends Regulation (EU) 2017/746 on the...
Validity of ISO 13485 QMS certificates after Brexit
Medenvolve Consultancy2023-12-22T11:06:56+03:00A quality management system (QMS) based on the ISO 13485 standard is an internationally recognised model that a...