 Product Tracking System (ÜTS for short), MDR, EUDAMED are among the topics that the medical device industry has been thinking about recently. In this article, we aim to inform you about frequently asked questions, especially in the context of these three titles (ÜTS, MDR, EUDAMED), which are closely related to the medical device industry. Let’s start!
Product Tracking System (ÜTS for short), MDR, EUDAMED are among the topics that the medical device industry has been thinking about recently. In this article, we aim to inform you about frequently asked questions, especially in the context of these three titles (ÜTS, MDR, EUDAMED), which are closely related to the medical device industry. Let’s start!
Has the Product Tracking System (ÜTS) changed recently? What can we do or what should we do about it?
Since the MDR (ie new Medical Device Regulation) came into force, some radical changes have occurred in terms of UTS (Product Tracking System). As the first and most important of these changes, we can specify the “Basic UDI” information. Basic UDI information took its place among the mandatory information that should be stated on the Declaration of Conformity. In general, there have been no other fundamental changes in the Declaration of Conformity that we have mentioned in the context of ÜTS, MDR, EUDAMED, if you are planning to prepare or prepare a Declaration of Conformity duly, you can contact us.
Have the EC Certificate obligations changed? How should our EC Certificates be in terms of ÜTS, MDR, EUDAMED?
As is known, Notified Bodies issue EC Certificates. This is an ongoing criterion for ÜTS, MDR and EUDAMED. That is, EC Certificates are still issued by the appointed Notified Bodies. You can find which Notified Bodies are accredited for ÜTS, MDR, EUDAMED from this list. Apart from this, if there is no medical device class change, there is no EC Certificate requirement for Class I devices (except sterile ones).
Is EUDAMED registration mandatory for us? How should we register EUDAMED?
EUDAMED (European Database on Medical Devices) was gradually opened to the role of an actor. It is a system in which a limited number of producers/actors from Turkiye have registered so far, and it will be necessary to register gradually for the actors in our country. For the registration created by binding on a contract, the person responsible for the legislation must first be appointed on the ÜTS. Then the sequence of operations is completed. If your registration for UTS has been completed without any problems, your EUDAMED registration should be completed quickly. If you want to learn more about this technical subject, you can contact us immediately.
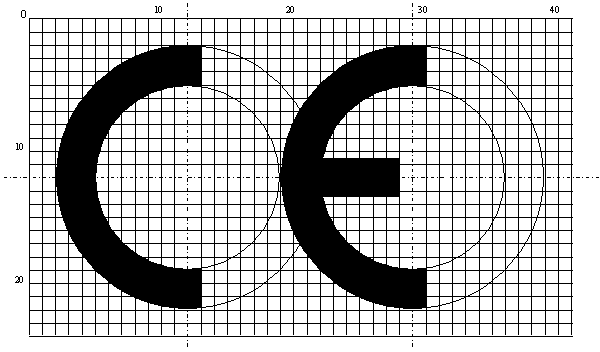
What is CE Marking? How should we use my AND sign? Is the CE mark mandatory for our device?
The CE mark generally indicates that a product is produced in accordance with European standards and that the use of the relevant product is not objectionable in terms of human health. In this context, the CE mark should definitely be used on medical devices. Medical devices that do not carry the CE mark cannot be placed on the market. Therefore, the CE mark on your product is mandatory.
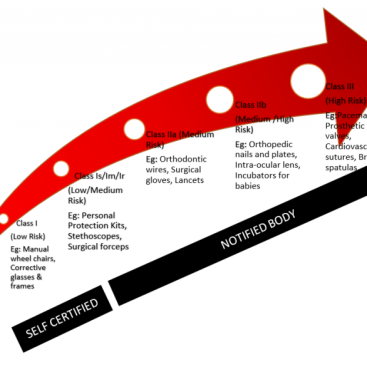
If you have a Class I (non-sterile) product, you can apply the CE Mark to your product depending on your own declaration, while in other classes you must be certified by the Notified Body.
How can we access the basic UDI code? What can be done if this code is not found on the declaration of conformity?
The basic UDI code is among the mandatory information on the declarations of conformity at the time of writing (02.2022). This information should be given by the manufacturer. You should contact your manufacturer of basic UDI knowledge. If your manufacturer does not have a study on this subject yet, you can remind your manufacturer of MDR obligations. In this regard, we can assist you in providing the appropriate Basic UDI information by informing your manufacturer. If there is no Basic UDI information on the Declaration of Conformity, your certificate cannot be registered to the ÜTS.
Is UTS registration mandatory when EUDAMED registration is made?
In fact, we can say that ÜTS and EUDAMED are similar to each other and even integrated at some points. However, both registrations are mandatory. Since UTS records are also used in SGK transactions, you should definitely make UTS registrations. Having a EUDAMED registration does not remove your UTS registration obligation.
Is the GMDN Code still valid? What can we do for the inactive GMDN code?
The GMDN Code is necessary and mandatory information for all medical devices. These codes, which are defined by an organization with the title of GMDN Agency, must be provided up-to-date and entered into the system during the ÜTS product registration process. Do not forget that; GMDN Codes must be available in the UTS database up-to-date. If you do not have a GMDN code registered on the ÜTS for your product that you will launch, you can contact us and request support for obtaining the GMDN code.