SSCP = Summary of Safety and Clinical Performance
The Safety and Clinical Performance Summary is to provide healthcare professionals with: (a) summary of clinical evaluation results of all available clinical data regarding the device, (b) provide information about the safety and performance of the device, including its clinical benefits and the success rate to achieve the intended results, (c) Inform patients of residual risks and side effects with Class III and implantable devices intended for direct patient use.Contents in Summary of Safety and Clinical Performance
- ID of device and manufacturer, including basic UDI-DI (Unique Device ID – device identifier) and SRN (single registration number) if previously issued: The first part of the SSCP should identify the device and manufacturer, The Product ID also contains some general information about the device.
- Intended use of the device and any indications, contraindications, and target populations: The purpose and indications should be described, including the stages or severity of pathologies, specific medical conditions and specific anatomical sites, or confirmation that no anatomical site is present. contraindicated as appropriate. The target audience will be specified.
- A description of the device, including a reference to previous generation or variants, if any, and a description of the differences, as well as, where relevant, a description of all accessories, other devices and products intended for use with the device: Description of the device, its working principles and how it works, Design features will contain information on whether the device is disposable or not.
- Possible diagnosis or treatment alternatives.
- Reference to harmonized standards and applied CS: A list will be provided containing all applied common specifications (CS), harmonized international standards and Non-Harmonized standards, and relevant monographs of the European Pharmacopoeia. Summary of clinical evaluation and relevant information on post-market clinical follow-up as in Annex XIV in MDR: This section is intended to comprehensively summarize clinical evaluation results and clinical data constituting clinical evidence for confirmation. compliance with relevant general safety and performance requirements, assessment of undesirable side effects, and acceptability of the benefit-risk ratio.
- The SSCP should include a statement if the conformity of the device has been evaluated and approved by the NB on an equivalence basis. If equivalence is used, it should be identified by the name of the device(s) for which the equivalence is shown, and the Basic UDI-DI, if any, and the name(s) of the manufacturer(s).
- All clinical studies conducted before the CE marking of the device in question should be summarized.
- If available, a summary of other clinical data on the device itself and key findings should be included. An overall summary of clinical performance and safety should be provided and supported by clinical evidence.
- Recommended profile and training for users: The experience, training or education of target users will be disclosed. This includes any special mandatory training prior to using the device and any updated training for continued safe use of the device.
- Information on any residual risks and undesirable effects, warnings and precautions.
When and Where Can SSCP Be Applied?
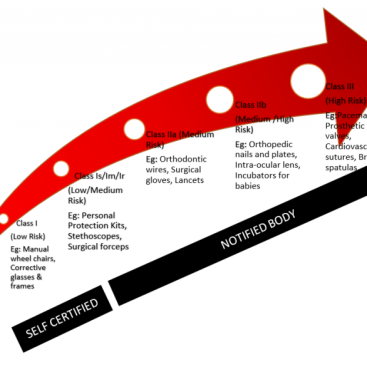
- The summary of safety and clinical performance (SSCP) set by the EU’s Medical Devices Regulation 2017/745 applies to implantable devices and class III devices that are not bespoke or investigational. (Ex: All implantable devices intended to be placed on teeth, Long-term surgically invasive devices intended for use in direct contact with the heart, central circulatory system or central nervous system)
- The SSCP, which will be verified by a notified body, will be made publicly available through the EU database, Eudamed, which is expected to be an important source of information for device users and healthcare professionals. The SSCP is not intended to provide general advice on the diagnosis or treatment of certain medical conditions, or to replace the instructions for use (IFU) as the main document ensuring the safe use of a device, or replace the mandatory information on implant cards or implant cards. other documents.
- The Safety and Clinical Performance Summary is currently approved and Notified Body verified and validated clinical data on the safety and clinical performance of a medical device for healthcare professionals and patients. The information provided in the SSCP should originate from the technical documentation of the device, which should be fully detailed, systematic and objective.
- It is useful to validate the pre-market and post-market studies suggested by the device, understand the strength and weakness of another similar device, and validate the risk.
- SSCP removes all residual risks and unwanted side effects.
- It is easy to understand for target users and easily accessible for both patients and Healthcare professionals once uploaded by the Notified body to the EUDAMED Site. The data is transparent and available in English as well as in the languages of the Member States where the Device is made available.
- The Safety and Clinical Performance Summary provides, among other information, an objective and balanced summary of clinical evaluation results regarding whether all available clinical data regarding the device in question are positive, negative and/or inconclusive.