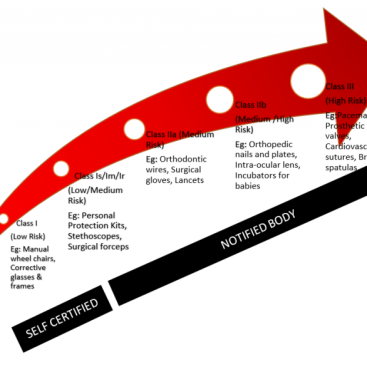
As of 26 May 2021, the European Union applies stricter rules on Medical Devices (MDR). From 26 May 2022, the new regulations will also apply to the In Vitro Diagnostic (IVDR) Regulations.
Parties, such as distributors and importers, have certain obligations that they and the device must meet in order to be compatible.
Let’s Install Your System to the extent of the obligations brought by this new regulation as a distributor or importer.
Let us help you manage your process within the scope of MDR and IVDR Article 13 and Article 16.
Requirements for MDR / IVDR Importers
Are you importing devices from outside the European Union to the European market? Then the new MDR regulations apply to you too. Let us ensure that you register with EUDAMED on time. It is important to note that in some cases the manufacturer, distributor and importer may all be the same company. In this case, the company will have to meet the obligations of all three parties.
However, this is not always the case, and sometimes a company may only play one or both of the roles. It may be difficult to understand the roles in the regulations, MedENvolve Consultancy will be your solution partner in fulfilling these obligations in order to understand the roles in the recently changed regulations and to manage your process without risk.
MedENvolve Consultancy provides Consultancy services in System Installation and EUDAMED registration processes in New MDR and IVDR processes for MDR and IVDR Distributor and Importer companies. Call us at 05447782036, email us at info@medenvolve.com, get in touch with our expert team today.
MDR/ IVDR : Requirements for Distributor
The new European Union directive for medical devices, EU MDR 2017/745, has just entered into force and interested parties are still working to transition from the old MDD 93/42/EEC regulation. You can browse our site to learn more about how the regulation is implemented. An important aspect of regulation is the distinction that a product makes between interested parties in the supply chain, including manufacturers, distributors, importers and authorized representatives. While most of the MDR now applies to manufacturers, certain sections of the regulation detail the specific responsibilities each party will assume.
The transition to EU MDR 2017/745 and IVDR 2017/746 is a huge challenge for many companies.
Understanding the role played is key to a successful transition.
MedENvolve Consultancy helps you understand the new regulation, make sure that your company and your device go through processes that comply with the regulation, and register your device in EUDAMED.