Companies producing Medical Devices should appoint an experienced ISO 13485 Consultant or a consulting firm with knowledge in all risk class devices. Legal requirements in the medical device industry are constantly reviewed and amended accordingly, with an increasing emphasis on patient safety.
Firms must manage and extract value from large volumes of structured and unstructured information and data, ensuring that their systems remain up-to-date and comply with regulatory, regional and local requirements. Firms must implement effective systems to monitor and monitor safety and benefit-risk information throughout the lifecycle of their products in accordance with ISO 13485 requirements.
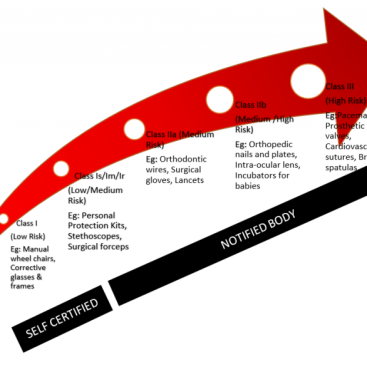
ISO 13485 Consultant can define and integrate product specific regulatory requirements such as MDR or IVDR or FDA 510k during implementation. This will be helpful later when the organization plans to obtain a CE Mark or 510k Customs Clearance.
Role and Responsibility of ISO 13485 Consultant
ISO Consultants assist clients with expert guidance in the planning, development and implementation of the QMS:
- Determination of QMS standard requirements
- Identification of all legal and regulatory requirements based on product classification
- Planning to intelligently build the standard requirement phase
- Developing and defining processes and documentation
- Training the QMS team
- Implementation of the QMS
- Conducting Internal Quality Audits
- Organizing Management Review Meetings
- Developing a continuous improvement approach in the entire QMS
- Evaluate the effectiveness of the QMS implemented in the organization
ISO 13485 Quality Manual
The Quality Manual is considered the main document in a QMS system designed for medical device manufacturers. If you manufacture CE Marked medical devices for use in the EU region, you will need to prove compliance with EN ISO 13485:2016. ISO 13485 provides flexibility in how organizations choose the structure of the Quality Manual or how it is distributed.

ISO 13485 Mandatory Procedures
The organization to which ISO 13485 applies may have many types of procedures or Standard Operating Procedures or Work Instructions or Protocols based on processes and activities.
Throughout the standard, ISO 13485:2016 has used words such as “should, must, mandatory, documented procedures” that directly warn the organization to keep documentation in the form of procedures, in more than one place. These procedures are often called mandatory procedures.
ISO 13485:2016 & PDCA cycle (Plan – Do – Check – Act)
ISO 13485:2016 follows the PDCA cycle (Plan – Do – Check – Act) which helps the organization adopt a drive for continued excellence in its processes. This approach can improve any process by breaking it down into smaller steps and is effective for the implementation of the QMS and helps improve the entire product realization process.
It also provides a set of solutions to problems and helps to choose the best one for the application. This method helps to allocate resources appropriately, avoiding waste for successful implementation of solutions.