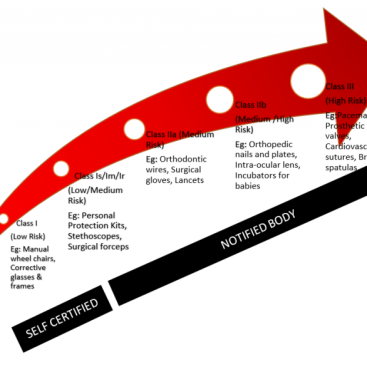
Clinical evaluation is required for medical devices under medical device directive 93/42/EEC and medical device regulation 2017/745 and even to some extent 2017/746 (for in vitro diagnostic devices). There are also many guidelines for clinical evaluation in the context of CE marking: MEDDEV 2.7.1 is the most important.
Clinical evaluation is the evaluation and analysis of clinical data on a medical device to confirm its clinical safety and performance. Evaluation is based on comprehensive analysis of pre- and post-marketing clinical data relevant to the intended use. This includes device-specific data as well as all data related to devices claimed to be equivalent by the manufacturer. The entire process is documented in a clinical evaluation report (CER).
For this purpose, the following data sample can be prepared:
- Clinical Studies
- Literature search and review
- Equivalent product data (literature and adverse events) to demonstrate safety and performance
- Clinical experience (implant records etc…)
- Post-market data
We should distinguish the following aspects:
- State of the art: how to position the device or technology in the light of current knowledge and available products. This is usually done by conducting a literature search for the usage indicator with the last time window.
- Safety and performance claims: it is important to identify all available literature for the product itself and the equivalent product to demonstrate that the claims are well supported.
The Clinical Evaluation Report (CER) documents the results of the clinical evaluation of your medical device. A CER consists of analyzed clinical data collected from a clinical study of your device or the results of other studies conducted on substantially equivalent devices. CER indicates that your device has achieved its intended purpose without exposing users and patients to further risk.
Clinical Evaluation Reports are required for all medical devices in Europe. You must submit your CER to your Notified Body as an annex to your European CE Technical File. The Technical File is an important step in obtaining the CE Mark for your device, which is required for selling or distributing medical devices in Europe.
How to prepare a Clinical Evaluation Report for medical devices
Clinical evaluation takes place in three steps. In step one, manufacturers identify clinical data from available literature, clinical experience, clinical trials, or any combination of the three. The second phase includes evaluating the relevance, feasibility, quality, and importance of vHow to prepare a Clinical Evaluation Report for medical devices. The third step requires you to express your results in the CER based on the data you have collected.
Treat CER as a standalone document, even if you’re going to include it in your technical or design file. Here is a list of possible items to include in your CER:
- General information: device and manufacturer name
- Brief physical and technical device description and intended application
- Outline of intended therapeutic or diagnostic claims
- Cutting-edge discussion
- Clinical assessment and data types
- Summary and review of clinical data
- Identify analyzes used to evaluate performance, safety, and relevance/accuracy of product literature
- Conclusions on safety, performance, and compliance
This is also supported by a literature search protocol and report and post-market data.
Required updates for your Clinical Evaluation Report
Regular updates to your CER are required as part of your post-market surveillance and vigilance activities. You should save any significant changes that affect the initial data and add the CER accordingly. Failure to do so may jeopardize your compliance with the Medical Devices Directive.
Europe’s new Medical Device Regulation (MDR) will introduce even more stringent requirements for Clinical Evaluation Reports, such as the basis for establishing equivalence with other devices and the quality of the data considered in your clinical evaluation.
Our Services
Our services include:
- Consultation: best strategy to develop clinical assessment plan
- Review of the latest technology
- Post-market data preparation
- Preparation of literature search protocol
- Preparation of literature research report
- Performance and security analysis and critical evaluation
- Link to risk assessment
- Preparation of the Clinical Evaluation Report: an overview of all of the above