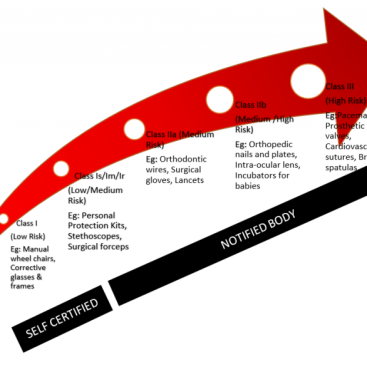
 The MDR 2017 / 745 Annex II Medical Device Technical File is a clear, well-organized, easily searchable and clearly prepared summary document by the manufacturer to demonstrate the safety and performance of the device in question.
The MDR 2017 / 745 Annex II Medical Device Technical File is a clear, well-organized, easily searchable and clearly prepared summary document by the manufacturer to demonstrate the safety and performance of the device in question.
The MDR technical file template must be submitted to the Notified Body or Competent Authority for review and approval. It should preferably be done in English or the official language of an EU Member State. It should be available on demand for the entire lifecycle of the medical device (5 years for low-risk devices and 16 years for high-risk devices). Non-EU manufacturers must keep the file at the authorized representative in the EU.
Regardless of the medical device class, intended use, construction and or the device’s safe history, the manufacturer must maintain and maintain up-to-date medical device technical file documents demonstrating compliance with general safety and performance requirements, MDR 2017 Annex I /745.
Technical File Selection Criteria
Quality Assurance and Regulatory team members are always confused or difficult to determine the required number of medical device technical files for products planned for CE Marking.
It is recommended that you remember that the medical device technical file or documentation is all about your device, to establish safety and performance. It means that you cannot combine products with different purposes, different classes, different building materials, or even different designs.
Medical Device Technical File Consultant
An experienced professional in EU Medical Device CE Marking covering key technical areas such as risk analysis, validations, design, shelf life, usability, biological evaluation, safety, chemical properties and clinical evaluation is called an expert. To prepare a technical file, you need an expert, that is, a consultant. We can help you in this difficult process, you can contact us directly for technical file preparation.
- Requirement and Description in accordance with CE MDR
- Determination of risk classification and assessment path for CE Marking
- Organizing and reviewing files systematically
- Clinical Assessment documents covering PMS, PMCF and PSUR
- Risk analysis
- Medical Device Quality Management System Application
Medical Device Technical File Types
MDR has set specific requirements for what to include in your technical documentation.
|
SR. NO. |
TECHNICAL FILE TYPE |
APPLIED RULES |
CONFORMITY |
|
CLASS I |
|||
|
1. |
CLASS INon-Invasive Device | Rule-1 All Non-Invasive devices are classified as class I | Conformity Assessment Article 52 (7). Manufacturers of class I devices, other than custom-made or investigational devices, declare the conformity of their products by issuing the EU declaration of conformity referred to in Article 19, after preparing the technical documents referred to in Annexes II and III.Notified Body participation is not mandatory.
CE marking can be affixed with its own declaration |
| Rule 2 – Any non-invasive device designed to channel or store blood, body fluids, cells or tissues, fluids or gases for the purpose of eventual infusion, administration or entry into the body, not affiliated with a class IIa, class IIb or class III active device; or is in class I, which is not intended to be used to channel or store blood or other body fluids, or to store organs, organ parts, or body cells and tissues. | |||
| Rule 4 – All non-invasive devices that come into contact with injured skin or mucous membranes if intended for use as a mechanical barrier, for compression or for resorption of exudates. | |||
|
2. |
CLASS IInvasive Device | Rule 5 – Except for surgically invasive devices, all invasive devices pertaining to body openings that are not intended or intended to be attached to an active device are Class I if they are intended for temporary use. (‘Temporary’ normally means designed for less than 60 minutes of continuous use.) | |
| Rule 5- A device used in the oral cavity up to the pharynx, in the ear canal or nasal cavity up to the eardrum and not absorbed by the mucous membrane | |||
|
3. |
CLASS Iactive device | Rule 10 – Active devices intended to illuminate the patient’s body in the visible spectrum are then classified as class I. | |
| All other software except those specified under Rule 11- Class IIa, Class IIb and Class III active device are classified as Class I. | |||
| Rule 13- All other active devices are classified as class I. | |||
|
4. |
class Isterile and
measuring instrument |
Article 52 (Article 7) | Conformity Assessment Article 52 (7). For Reusable Surgical Instruments, the manufacturer shall comply with Annexes I and III of Annex IX. will administer the CA according to the procedures set out in Chapters or Part A of Annex XI.Participation of the notified body in the following cases:
(a) in the case of devices placed on the market in sterile condition, aspects of establishing, securing and maintaining sterile conditions. (b) in the case of devices with a measuring function, considerations regarding the compliance of the devices with metrological requirements. Notified body participation is mandatory for sterile medical device CE marking and the device must comply with the Quality Management System. – Measuring devices supplied in sterile condition require notified body certification, non-sterile device does not require notified body involvement. |
|
5. |
CLASS ISurgical Invasive Device | Rule 6 – All surgically invasive devices intended for temporary use are reusable surgical instruments | Conformity Assessment Article 52 (7). For Reusable Surgical Instruments, the manufacturer shall comply with Annexes I and III of Annex IX. will administer the CA according to the procedures set out in Chapters or Part A of Annex XI.Participation of the notified body in matters relating to device reuse, cleaning, disinfection, sterilization, maintenance and functional testing and associated instructions for use, in the case of reusable surgical instruments. |
|
Class IIa |
|||
|
6. |
CLASS IIaNon-Invasive Device | Rule 2 – All non-invasive devices designed to channel or store blood, body fluids, cells or tissues, liquids or gases for final infusion, administration or introduction are classified as class IIa if they are subject to: A class IIa, Class IIb or class III active device or these shall be Class IIa if they are intended to be used to divert or store blood or other body fluids or to store organs, organ parts or body cells and tissues. | Conformity Assessment Article 52 (6). Manufacturers of class IIa devices, other than custom-made or investigational devices, shall comply with Annexes I and III of Annex IX. at least one representative device for each device category.OR
The manufacturer may choose to prepare the technical documents specified in Annexes II and III together with a conformity assessment as specified in Annex XI Section 10 or Section 18. Evaluation of technical documentation will be valid for at least one representative device for each device category. Declaration of Conformity with Notified body assessment before affixing the CE marking |
| Rule 3 – All non-invasive devices intended to change the biological or chemical composition of human tissues or cells, blood, other body fluids or other fluids intended for use, the treatment in which the device is used consists of filtration, centrifugation or gas exchange, temperature | |||
| Rule 4 – All non-invasive devices that meet injured skin or mucous membranes, if mainly intended for managing the microenvironment of injured skin or mucous membrane | |||
|
7. |
CLASS IIaInvasive Device | Rule 5 – Except for surgically invasive devices, all invasive devices relating to body openings that are not intended for or intended to be connected to an active device are class IIa if they are designed for short-term use. term use (‘Short term’ means normally intended for continuous use between 60 minutes and 30 days) | |
| Rule -5 A device used in the oral cavity up to the pharynx, in the ear canal or nasal cavity up to the eardrum and which is not subject to absorption by the mucous membrane is then classified as class IIa | |||
| Rule 5 – All invasive devices are classified as class IIa based on body openings other than surgically invasive devices intended for attachment to a class IIa, class IIb or class III active device. | |||
|
8. |
CLASS IIaSurgical Invasive Device | Rule 6 – All surgically invasive devices intended for temporary use are classified as class IIa | |
| Rule 7 – All surgically invasive devices intended for short-term use are classified as class IIa (‘Short-term’ means normally intended for continuous use between 60 minutes and 30 days) | |||
|
9. |
CLASS IIa |
Rule 8- All implantable devices and long-term surgically invasive devices intended for placement on teeth. | |
|
10. |
CLASS IIaActive Therapeutic Device | Rule 9- All active therapeutic devices designed to energize or exchange energy are classified as class IIa. | |
|
11. |
CLASS IIaActive Device | Rule 10 – Active devices for diagnostic and monitoring purposes are class IIaRule 10 Active devices, if they are intended to provide energy to be absorbed by the human body
Rule 10 Active devices, if intended to monitor the in vivo distribution of radiopharmaceuticals. Rule 10 Active devices intended to allow direct diagnosis or monitoring of vital physiological processes |
|
| Rule 11 – Software intended to provide information used to make diagnostic or therapeutic decisions is class IIa,Rule 11- Software for monitoring physiological processes is classified as class IIa. | |||
| Rule 12- All active devices designed to introduce and/or remove medicinal products, bodily fluids or other substances into or from the body are classified as class IIa. | |||
|
12. |
CLASS IIaSpecial case | Rule 16- All devices intended to be used specifically for the disinfection or sterilization of medical devices are classified as class IIa. | |
| Rule 17- Devices specially designed for recording diagnostic images produced by X-ray radiation are classified as class IIa. | |||
| Rule 19 – All devices containing or composed of nanomaterials are class IIa if there is a negligible potential for internal exposure. | |||
| Rule 20 – All invasive devices based on body openings are classified as class IIa, except surgically invasive devices designed to deliver medicinal products by inhalation. | |||
| Rule 21. Devices consisting of substances or combinations of substances intended to be administered to the human body through a body puncture or applied to the skin, andThose that are absorbed or locally dispersed by the human body are classified as class IIa when applied to the skin or applied in the nasal or oral cavity up to the pharynx and achieve their intended purpose in these cavities. | |||
|
CLASS IIb |
|||
|
13. |
CLASS IIbNon-Invasive Device | Rule 2 – Non-Invasive – Blood Bags | |
| Rule 3 All non-invasive devices intended to alter the biological or chemical composition of human tissues or cells, blood, other body fluids, or other fluids intended to be implanted or delivered into the body are classified as class IIb. | Conformity Assessment Article 52 (4). Manufacturers of class IIb devices, other than custom-made or investigational devices, are subject to Articles I and III of Annex IX. one representative device per generic device groupOR
The manufacturer may choose to apply a conformity assessment based on the type examination specified in Annex X, together with a conformity assessment based on product conformity verification specified in Annex XI. For all class IIb devices referred to in point (b) of Article 61:-54(1), the manufacturer may consult a panel of experts referred to in Article 106 prior to clinical evaluation and/or research. purpose of reviewing the manufacturer’s intended clinical development strategy and clinical trial recommendations. The manufacturer will give due consideration to the views expressed by the expert panel. Conformity Assessments are required before the CE marking can be affixed by the Notified Body. Review of the QMS implementation, Technical file, Clinical Evaluation, Design review by the notified body. |
||
| Rule 4 Any non-invasive device that meets the injured skin or mucous membrane if it is intended to be used primarily for skin injuries that rupture the dermis or mucous membrane and can only heal with secondary intent. | |||
|
14. |
CLASS IIbInvasive device | Rule 6- Except for surgically invasive devices, all invasive devices pertaining to body openings that are not intended for or intended to be connected to an active device are Class IIb if they are designed for long-term use. term use (‘Long term’ normally means intended for continuous use of more than 30 days.) | |
|
15. |
CLASS IIbSurgical Invasive device | Rule 6 All surgically invasive devices intended to deliver energy in the form of ionizing radiation are then classified as class IIb.Rule 6 – All surgically invasive devices have a biological effect or are completely or largely absorbed, classified as class IIb
Rule 6- All surgically invasive devices intended to deliver medicinal products through a delivery system are class IIb if such administration of a medicinal product is potentially dangerous given the mode of administration. |
|
| Rule 7 – Any surgically invasive device intended to provide energy in the form of ionizing radiationRule 7 – All surgically invasive devices for drug delivery,
Rule 7 – All surgically invasive devices intended to undergo chemical changes in the body, except for the placement of devices on the teeth |
|||
|
16. |
CLASS IIbActive Therapeutic Device | Rule-9 All active therapeutic devices in which they may apply or exchange energy with the human body in a potentially dangerous manner, taking into account the nature, intensity and site of application of the energy, are class IIb. | |
|
17. |
CLASS IIbActive Device | Rule 9 – All active devices intended to control or monitor the performance of active therapeutic class IIb devices, or intended to directly affect the performance of such devices, are classified as class IIb.Rule 9- All active devices intended to emit ionizing radiation for therapeutic purposes, including devices that control or monitor such devices or directly affect their performance, are classified as class IIb. | |
| Rule 10 – Active devices for monitoring vital physiological parameters and the nature of their variations are for diagnostic purposes, for example in cardiac performance, respiration, central nervous system activity, or clinical situations where the patient is in immediate danger, and are classified as class IIb in these situations.Rule 10 – Active radiological diagnostic or therapeutic devices intended to emit ionizing radiation, including interventional radiology devices and devices that control or monitor these devices or directly affect their performance, are classified as class IIb. | |||
| Rule 11- Software intended to provide information used to make diagnostic or therapeutic decisions that may affect a serious deterioration in a person’s health or surgical intervention is classified as class IIb.Rule 11- Software for monitoring vital physiological parameters is classified as class IIb if the nature of the variations of these parameters is such that they may present an immediate danger to the patient. | |||
| Rule 12- All active devices designed to introduce and/or remove medicinal products, bodily fluids or other potentially hazardous substances into or from the body, the relevant substances, the relevant part of the body and the mode of administration, are then classified as class IIb. | |||
|
18. |
CLASS IIbSpecial Rules | Rule 15- All devices used for contraception or to prevent the transmission of sexually transmitted diseases are classified as class IIb. | |
| Rule 16- All devices intended for use specifically for disinfecting, cleaning, rinsing or, where appropriate, moistening contact lenses are classified as class IIb.Rule 16. Disinfection solutions or washer-disinfectors intended specifically for disinfection of invasive devices as the end point of the process are then classified as class IIb. | |||
| Rule 19 – All devices containing or composed of nanomaterials if they have a low potential for internal exposure | |||
| Rule 20 – All invasive devices involving body openings, except surgically invasive devices intended to deliver medicinal products by inhalation, unless their mode of action has a significant impact on the efficacy and safety of the administered medicinal product. classified as class IIb, intended to treat life-threatening conditions | |||
| Rule 21- Devices consisting of substances or combinations of substances intended to be administered to the human body through a body puncture or applied to the skin and which are absorbed by the human body or dispersed locally within the human body are classified as class IIb. Class III, Class IIA In all other cases except as specified in Special Case | |||
|
19. |
CLASS IIb |
Rule 8- Whole implantable devices and long-term surgically invasive devices are classified as class IIb (‘Long-term’ means intended for continuous use normally greater than 30 days.) | Conformity Assessment Article 52 (4). For class IIb implantable devices, with the exception of sutures, staples, dental fillings, braces, dental crowns, screws, wedges, plates, wires, pins, clips and connectors, the assessment of the technical documentation specified in Annex IX Section 4 shall apply. for each device. |
|
CLASS III |
|||
|
20. |
CLASS III- Non-Invasive Device |
Rule 3 – All non-invasive devices consisting of a substance or mixture of substances taken from the human body prior to implantation or administration, or intended for use in vitro in direct contact with human cells, tissues or organs used in vitro with human embryos. | Conformity Assessment: – Article 52 (3) Manufacturers of class III devices other than bespoke or investigational devices are subject to a conformity assessment as specified in Annex IX. Alternatively, the manufacturer may choose to apply a conformity assessment as specified in Annex X together with a conformity assessment as specified in Annex XI.Notified body inspection device design, technical file according to Annex II before adding the CE logo.
For Rule 14: In addition to CA Article 52 (3) above, the Procedure set out in Section 5.2 of Annex IX or Section 6 of Annex X will also apply. For Rule 18:- In addition to CA Article 52 (3) above, the Procedure set out in Section 5.3 of Annex IX or Section 6 of Annex X will also apply. Article 61(2)- For all class III devices referred to in point (b) of Article 54(1), prior to clinical evaluation and/or research, the manufacturer may consult an expert panel referred to in Article 106, the manufacturer’s intended clinical development to review strategy and clinical trial recommendations. The manufacturer will give due consideration to the views expressed by the expert panel. |
|
21. |
CLASS III Surgical Invasive Device |
Rule 6- All surgically invasive devices are specifically designed to control, diagnose, monitor or correct a defect in the heart or central circulatory system by direct contact with these parts of the body, in which case they are classified as class III; Rule 6- All surgically invasive devices are specifically designed for use in direct contact with the heart or the central circulatory system or the central nervous system, in which case they are classified as class III. |
|
| Rule 7 – All surgically invasive devices designed specifically to control, diagnose, monitor or correct a defect in the heart or central circulatory system by direct contact with these parts of the bodyRule 7 – All surgically invasive devices intended for use in direct contact with the heart or central circulatory system or central nervous system in particular
Rule 7 – All surgically invasive devices that have a biological effect or are completely or largely absorbed are classified as class III |
|||
|
22. |
Class III Implantable Device and Long Term Surgical Invasive Device |
Rule 8 – All implantable devices and long-term surgically invasive devices are class III if:1) Intended for use in direct contact with the heart, central circulatory system or central nervous system
2) has a biological effect or is completely or largely absorbed 3) Except for the devices placed on the teeth, it is aimed to undergo a chemical change in the body. 4) intended to administer medicinal products, 5) are active implantable devices or accessories. 6) breast implants or surgical nets, 7) are full or partial joint replacements, excluding auxiliary components such as screws, wedges, plates and tools. 8) spinal disc replacement implants or implantable devices that accommodate the spine, excluding components such as screws, wedges, plates, and instruments. |
|
|
23. |
Class III Active Device |
Rule 9- All active devices intended to control, monitor or directly influence the performance of active implantable devices are classified as class III. | |
| Rule 11-Software used to make diagnostic or therapeutic decisions, intended to provide information that has the effect of causing death or irreversible deterioration in a person’s health, then falls in class III; | |||
|
24. |
Class III Special Rules |
Rule 14-. All devices, including a medicinal product derived from human blood or human plasma, that contain as an integral part a substance that can be considered a medicinal product when used separately, and which have side effects of the devices are classified as class III. | |
| Rule 15 – Devices that are implantable or long-term invasive devices used for the prevention of transmission of sexually transmitted diseases or for contraception are classified as class III. | |||
| Rule 18 – All devices manufactured using tissues or cells of human or animal origin, or their derivatives, rendered inanimate or inanimate, are classified as class III unless they are devices intended to come into contact with intact skin only. | |||
| Rule 19 – All devices containing or composed of nanomaterials if they have a high or medium potential for internal exposure | |||
| Rule 21- Devices consisting of substances or combinations of substances intended to be administered to the human body through a body puncture or applied to the skin and which are absorbed by the human body or locally dispersed within the human body are class III, if any. or their metabolism products are systemically absorbed by the human body to achieve its intended purpose.Rule 21- Devices consisting of substances or combinations of substances intended to be administered to the human body through a body puncture or applied to the skin and which are absorbed by the human body or locally dispersed within the human body are class III, if any. they achieve their intended purpose in the stomach or lower gastrointestinal tract, and they or their products of metabolism are systemically absorbed by the human body; | |||
| Rule 22- Active therapeutic devices, such as closed-loop systems or automated external defibrillators, with an integrated or integrated diagnostic function that significantly determines patient management by the device, are class III. | |||
|
25. |
Systems and procedure packages |
Article 22Systems: – ‘system’ means a combination of products, packaged or unpackaged, that are intended to be interconnected or combined to achieve a particular medical purpose.
Procedure Packs: – ‘procedure pack’ means a combination of products that are packaged together and placed on the market for use for a specific medical purpose; |
|
| Paragraph 1. If natural or legal persons combine the devices bearing the CE mark with the other devices or products specified below in accordance with the intended use of the devices or other products and within the limits of use they have determined, they shall issue a declaration. To place them on the market as a system or procedure package, manufacturers must:(a) Other devices bearing the CE marking.
(b) In vitro diagnostic medical devices bearing the CE mark in accordance with Regulation (EU) 2017/746. (c) other products that comply with the legislation applicable to those products only where they are used in a medical procedure or where their presence in the system or procedure package is otherwise justified. |
Paragraph 2: In the statement made pursuant to paragraph 1, the relevant natural or legal person declares:(a) Verified the mutual compatibility of the devices and other products, if any, according to the manufacturers’ instructions and carried out their activities in accordance with these instructions.
(b) package the system or procedure package and provide users with relevant information, including information to be provided by the manufacturers of the assembled devices or other products. (c) The activity of combining devices and, if applicable, other products into a system or procedural package was subject to appropriate internal monitoring, verification and validation methods. Once the substance, system or package of procedures has been put together, it is made available to the competent authorities for the period applicable to the combined devices under Article 10(8). Where these periods differ, the longest period shall prevail. The system or procedure packages referred to in paragraph 1 of this article shall not by themselves carry an additional CE mark, but shall also bear the name, registered trade name or registered trademark of the person referred to in paragraphs 1 and 3 of this article. as the address where the person can be contacted so that the person’s whereabouts can be determined. |
||
| Paragraph 3: – The natural or legal person who sterilizes the systems or transaction packages referred to in paragraph 1 for placing on the market, | Follow one of the procedures set out in Annex IX or Part A of Annex XI, according to their preferences. The implementation of these procedures and the involvement of the notified body will be limited to aspects of the procedure relating to maintaining sterility until the sterile packaging has been opened or damaged. The natural or legal person draws up a declaration declaring that the sterilization was carried out in accordance with the manufacturer’s instructions. | ||
| Para4:-In cases where the system or procedure package contains devices that do not bear the CE marking, or the combination of devices selected is not compatible with their original intended use or sterilizations are not performed in accordance with the manufacturer’s instructions. Instructions. | The system or package of procedures will be treated as a device and will be subject to the relevant conformity assessment procedure in accordance with Article 52. The natural or legal person shall assume the obligations of the manufacturers. | ||
|
26. |
Custom Made Device |
EU MDR 2017/745 Annex XIII | The authorized representative prepares a statement set out in Annex XIII Section 1.Conformity Assessment: – Article 52 (8) Manufacturers of Class III custom-made implantable devices shall be subject to the conformity assessment set out in Annex IX Part I. Alternatively, the manufacturer may choose to apply a conformity assessment as specified in Annex XI Part A. |
|
27. |
Investigation devices |
Follow the requirements set out in clauses 62 to 81 | |