A medical device is not expected to evoke any biological response in the patient when used as intended and in contact with the patient’s body. Biological response, depending on the contact profile of the device with the body (contact tissue/body fluid and contact time);
- as well as on a cellular basis (cytotoxic)
- local (eg irritation/sensitization),
- systemic (eg acute, subacute/subchronic, or chronic toxicity) or
- can occur on a mutagenic (eg genotoxicity/carcinogenicity) scale.
In order to verify that Medical Devices do not have such effects, the devices are subjected to the biocompatibility evaluation process. The biocompatibility evaluation process is a multidimensional process that includes biological/chemical analysis tests performed on medical devices, scientific literature and animal tests, as described in detail in the ISO 10993 standard series. The biocompatibility data of the Medical Device is processed in the risk analysis in accordance with the ISO 14971 standard and is presented as evidence that it meets the relevant Basic Requirements set by the current Medical Device Regulation/Regulation.
Biological Evaluation
Biological Evaluation is a series of tests that can be performed on animal models with the help of international standards, preclinical both in-vitro and in-vivo techniques, in order to evaluate the biological safety of the medical device in the risk management process.
Biological Evaluation is done to measure the compatibility of a material with a biological system. Evaluation is carried out using a series of tests according to a standard corresponding to the product type, for example pharmaceuticals, cosmetics and medical devices, to demonstrate that the material or device will not pose any potential risk to humans during its use.
The primary purpose of biological evaluation is to protect the patient from the biological risk posed by the medical device.
Biological Evaluation Report
The Biological Evaluation Report is an aggregated summary of all biological tests performed and the reasons for the tests not performed. This includes supporting data from the literature, evaluation of the data, gap analysis for information currently available for biological safety, a rationale for why additional information is not required, and a statement confirming the completed biological risk analysis and risk controls.
The report is used to demonstrate that a medical device does not pose any potential risk to patients and target users during its use.
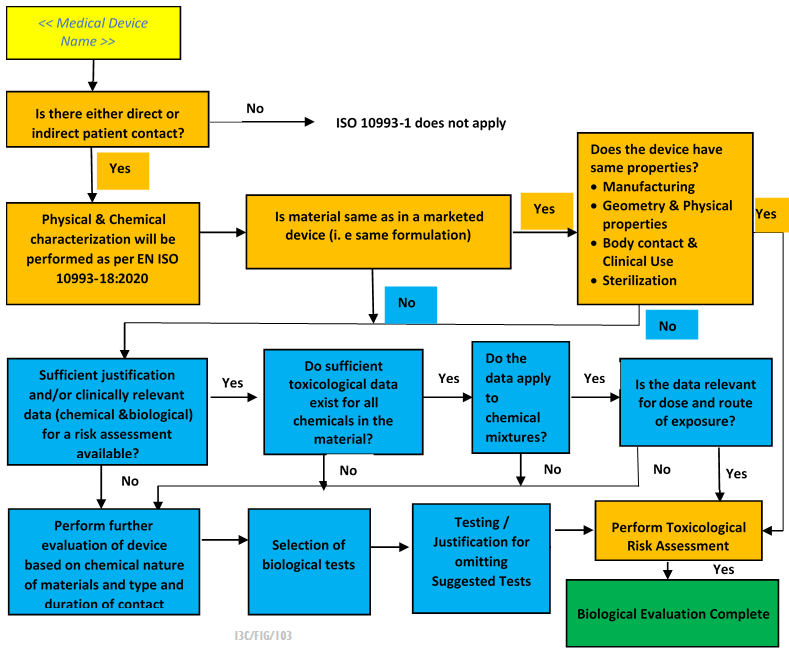
Biological Assessment Plan
A Biological Assessment Plan is used to demonstrate the safety of the device by demonstrating compliance with relevant standards and avoiding unnecessary testing of the device.
As biological assessment is a risk management activity, a Biological Assessment Plan is required and forms part of the Risk Management Plan.
The biological assessment plan highlights not only the biocompatibility testing but also the requirements of ISO 14971 risk management. The biological assessment plan should be prepared by a knowledgeable and experienced team and includes:
- Arrangements for collecting applicable information from published literature
- Arrangements for plan review and approval as part of the overall design control process
- Arrangements for reviewing the final results of the assessment and approval of any additional testing required
- Arrangements for final review and approval of the results of the biological risk assessment
Factors taken into account during Biological Evaluation
- Building material(s) (ie, all direct and indirect tissue contact materials);
- Medical device configuration (eg size, geometry, surface properties)
- Intended additives, process contaminants and residues during device manufacture
- Production processes
- Packaging materials that come into direct or indirect contact with the medical device
- Leakages and Decomposition products
- Other ingredients and their interactions in the final product
- Performance and properties of the final product
- Physical and chemical properties of the device
- Systemic effects and local effects of implantable devices
- Biohazards of each material and final product
- Available toxicology and other biological safety data